Formation of XPhos‐Ligated Palladium(0) Complexes and Reactivity in Oxidative Additions - Wagschal - 2019 - Chemistry – A European Journal - Wiley Online Library

trans-Dichlorobis(XPhos)palladium(II) Precatalyst for Suzuki–Miyaura Cross-Coupling Reactions of Aryl/Vinyl Sulfonates/Halides: Scope, Mechanistic Study, and Synthetic Applications | ACS Omega

Direct Evidence for Competitive C–H Activation by a Well-Defined Silver XPhos Complex in Palladium-Catalyzed C–H Functionalization - ScienceDirect

Palladium Extraction Following Metal-Catalyzed Reactions: Recent Advances and Applications in the Pharmaceutical Industry | Organic Process Research & Development
![Palladium(II) acetate/2-dicyclohexylphosphino-2,6-dimethoxy-1,1'-biphenyl (SPhos)/potassium phosphate admixture [CatKit single-use vials - 1.96 wt% Pd(OAc)2] | Strem Chemicals Palladium(II) acetate/2-dicyclohexylphosphino-2,6-dimethoxy-1,1'-biphenyl (SPhos)/potassium phosphate admixture [CatKit single-use vials - 1.96 wt% Pd(OAc)2] | Strem Chemicals](https://www.strem.com/uploads/web_structures/46-0395.gif)
Palladium(II) acetate/2-dicyclohexylphosphino-2,6-dimethoxy-1,1'-biphenyl (SPhos)/potassium phosphate admixture [CatKit single-use vials - 1.96 wt% Pd(OAc)2] | Strem Chemicals

Direct Evidence for Competitive C–H Activation by a Well-Defined Silver XPhos Complex in Palladium-Catalyzed C–H Functionalization - ScienceDirect

Formation of XPhos‐Ligated Palladium(0) Complexes and Reactivity in Oxidative Additions - Wagschal - 2019 - Chemistry – A European Journal - Wiley Online Library

Palladium Extraction Following Metal-Catalyzed Reactions: Recent Advances and Applications in the Pharmaceutical Industry | Organic Process Research & Development
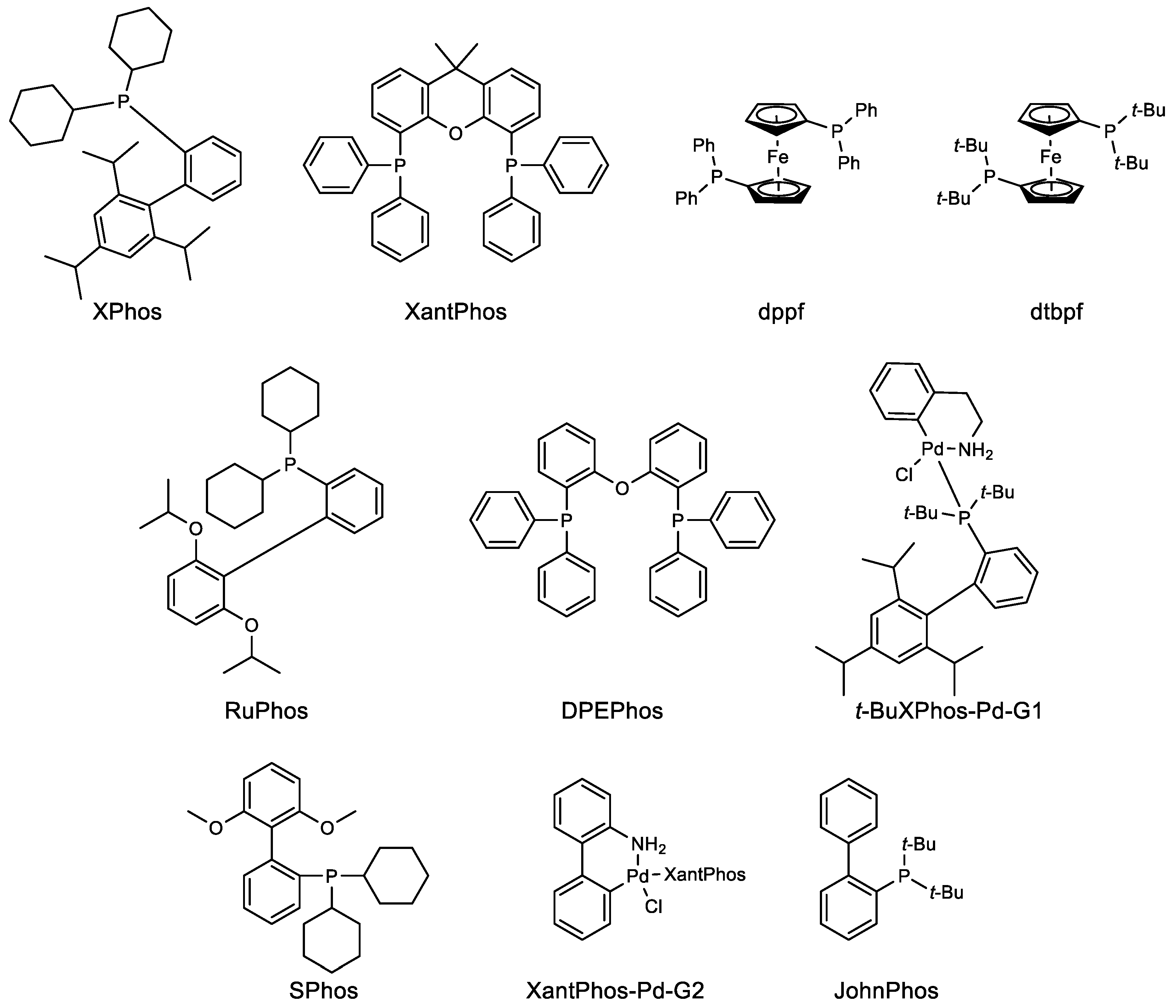
Organics | Free Full-Text | Recent Applications of Pd-Catalyzed Suzuki–Miyaura and Buchwald–Hartwig Couplings in Pharmaceutical Process Chemistry

Recyclable and Reusable Pd(OAc)2/XPhos–SO3Na/PEG-400/H2O System for Cyanation of Aryl Chlorides with Potassium Ferrocyanide | Catalysis Letters

Formation of XPhos‐Ligated Palladium(0) Complexes and Reactivity in Oxidative Additions - Wagschal - 2019 - Chemistry – A European Journal - Wiley Online Library

Palladium Extraction Following Metal-Catalyzed Reactions: Recent Advances and Applications in the Pharmaceutical Industry | Organic Process Research & Development
![PDF] Third Generation Buchwald Precatalysts with XPhos and RuPhos: Multigram Scale Synthesis, Solvent-Dependent Isomerization of XPhos Pd G3 and Quality Control by 1H- and 31P-NMR Spectroscopy | Semantic Scholar PDF] Third Generation Buchwald Precatalysts with XPhos and RuPhos: Multigram Scale Synthesis, Solvent-Dependent Isomerization of XPhos Pd G3 and Quality Control by 1H- and 31P-NMR Spectroscopy | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/4039b7f1dbfe96075162c56e3101b153795e53db/2-Figure1-1.png)
PDF] Third Generation Buchwald Precatalysts with XPhos and RuPhos: Multigram Scale Synthesis, Solvent-Dependent Isomerization of XPhos Pd G3 and Quality Control by 1H- and 31P-NMR Spectroscopy | Semantic Scholar

Formation of XPhos‐Ligated Palladium(0) Complexes and Reactivity in Oxidative Additions - Wagschal - 2019 - Chemistry – A European Journal - Wiley Online Library
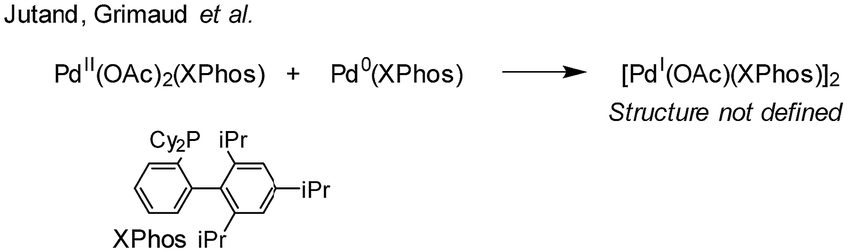
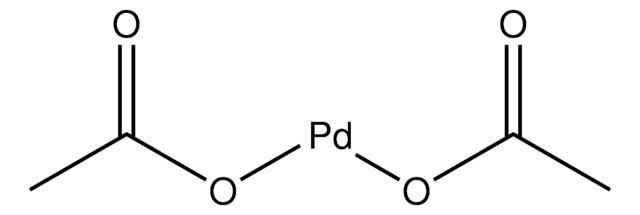
The ubiquitous cross-coupling catalyst system 'Pd(OAc) 2 '/2PPh 3 forms a unique dinuclear Pd I complex: an important entry point into catalytically c ... - Chemical Science (RSC Publishing) DOI:10.1039/C9SC01847F

Catalysts | Free Full-Text | Microwave-Assisted Palladium-Catalyzed Cross-Coupling Reactions: Generation of Carbon–Carbon Bond

trans-Dichlorobis(XPhos)palladium(II) Precatalyst for Suzuki–Miyaura Cross-Coupling Reactions of Aryl/Vinyl Sulfonates/Halides: Scope, Mechanistic Study, and Synthetic Applications | ACS Omega

TCI Practical Example: Buchwald-Hartwig Amination of Aryl Chlorides using a Palladium Catalyst and XPhos Ligand | TCI AMERICA
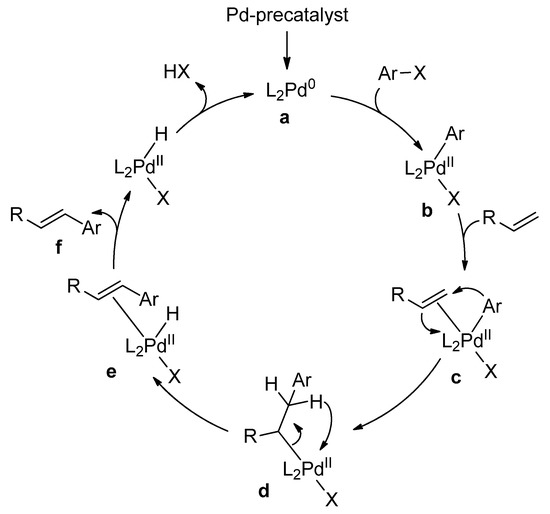
Catalysts | Free Full-Text | Microwave-Assisted Palladium-Catalyzed Cross-Coupling Reactions: Generation of Carbon–Carbon Bond

Synthesis of Differentially Protected Azatryptophan Analogs via Pd2(dba)3/ XPhos Catalyzed Negishi Coupling of N-Ts Azaindole Halides with Zinc Derivative from Fmoc-Protected tert-Butyl (R)-2-Amino-3-iodopropanoate | The Journal of Organic Chemistry